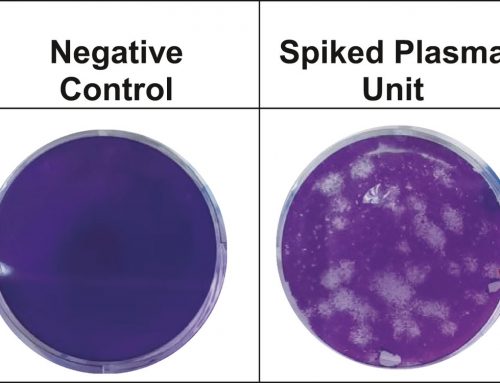
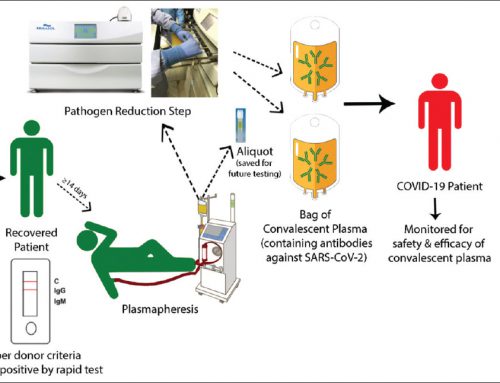
Chimeric antigen receptor (CAR) T-cell therapy represents a major advancement in personalized cancer treatment. In this strategy, a patient’s own T cells are genetically engineered to express a synthetic receptor that binds a tumor antigen.
CAR T cells are then expanded for clinical use and infused back into the patient’s body to attack and destroy chemotherapy-resistant cancer. Dramatic clinical responses and high rates of complete remission have been observed in the setting of CAR T-cell therapy of B-cell malignancies. This resulted in two recent FDA approvals of CAR T cells directed against the CD19 protein for treatment of acute lymphoblastic leukemia and diffuse large B-cell lymphoma.
CAR T cell therapies are amongst the first of a pipeline of cell therapies transitioning from ‘bench to bedside’ for both malignant and non-malignant diseases.
They are considered to be highly innovative personalized treatments offering potentially effective therapy with severe but manageable adverse events which require specialized monitoring and management. Indications for Advanced Cell Therapies are expected to expand beyond current indications, which are largely hematological malignancies. This will have implications for the teams involved and also where the treatment is administered.
Clinical application of CAR T cells is limited to a small number of manufacturing facilities (mainly located in the United State) with the required infrastructure and expertise for Good Manufacturing Practice (GMP). The manufacturing of CAR T cells is a complex process that can be summarized into the following steps : isolation of mononuclear cells from human subjects, activation of T cells to allow for viral transduction, expansion of cells and finally the harvest of the product.