AlShehry N, et.al
https://doi.org/10.4103/sjmms.sjmms_731_20
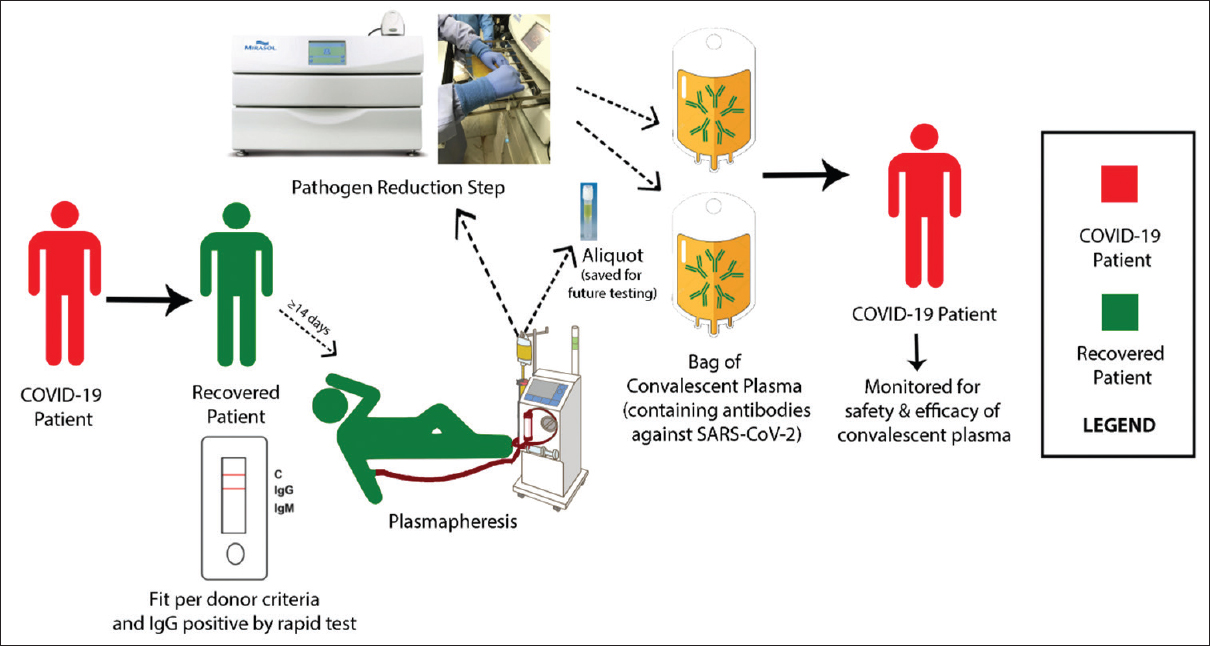
Al Shehry et al reported the interim findings of the Phase II trial that described the outcomes of 40 hospitalized adults with severe or critical COVID-19 infection who received convalescent plasma in comparison with 124 matched controls who did not receive convalescent plasma. There was no difference in the intensive care unit or hospital length of stay but there was an association between the use of convalescent plasma and lower risk of death with absolute difference in the risk of death of 13%.
Of note that, up to date, 5 randomized clinical trials with a total number of 960 have examined the effect of convalescent compared with controls not receiving convalescent plasma in COVID-19 patients. There was no safety concerns, however, none of the trials demonstrate a reduction in mortality in convalescent plasma.
The Saudi multicenter trial included more than 20 centers from Saudi Arabia.